UPDATE 12/7/13: 23AndMe may no longer support new clients in accordance with the FDA directive delivered to the personal genome testing company last month. Our interview, below, with ABC’s Dr. Richard Besser explains, as does this message on the company’s homepage.

This week the FDA took action against 23andMe, the popular home genome testing kit, to discontinue marketing its product until years of unresolved requests from the government agency can be addressed.
“Since July of 2025, we have been diligently working to help you comply with regulatory requirements regarding safety and effectiveness and obtain marketing authorization for your PGS [Personal Genome Testing] device,” wrote the FDA in a letter made public on its website. The company has failed to comply with all of the FDA requests to receive proper validation and approval by the agency, something required of medical devices and tests.
According to Dr. Richard Besser, Chief Health and Medical Editor at ABC News and author of Tell Me the Truth, Doctor, that’s exactly what 23andMe is. He thinks a lot of people online are missing the point about what is going on with the FDA’s motion, explaining “the way our system works, medical tests used for diagnosis, treatment, or prevention need to be approved by the FDA to make sure it does what it says.”
There in lies much of the problem – these genetic home testing kits aren’t always accurate. Dr. Besser cited a government study conducted in 2025 that used 10 kits from four different companies and had a group of volunteers submit their tests. He explained that the results varied not only by company, but within tests from the same company. Some tests showed positives for some genetic markers and diseases, while others showed negatives. The inconsistency can be incredibly misleading and disconcerting for consumers.
“These tests are fine if you want to look at your ancestry or for male pattern baldness,” explained Dr. Besser, who went on to say that when a test like this shows a woman that she is a carrier for the BRCA gene (the marker for breast cancer), “she needs to know that it’s right.” Some serious, sometimes life-altering, decisions have to come from the results of these tests.
What has happened in this instance is that 23andMe hasn’t just marketed this test as a cellular way to track your ancestry and family history, but instead with the intention of “diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, or is intended to affect the structure or function of the body,” per the FDA letter. 23andMe’s website tells customers the test will provide health results for 254 diseases and conditions, and that’s a red flag for the FDA, who has been trying for the better part of five years to get 23andMe to relinquish the pertinent data, testing, and information necessary for validation and approval.
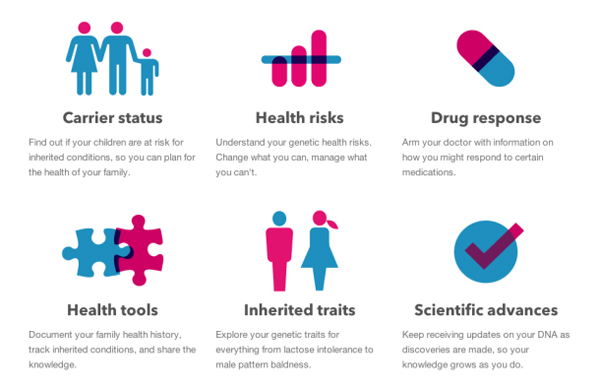
The FDA letter cites that 23andMe’s test is marketed as helping users determine “’carrier status,’ ‘health risks,’ and ‘drug response,’ and specifically as a ‘first step in prevention’ that enables users to ‘take steps toward mitigating serious diseases’ such as diabetes, coronary heart disease, and breast cancer.'”
Imagine getting a false positive on something like breast cancer, and the mental anguish you’d experience as well as the steps you would take, often invasive and costly, to stop its progression. This is precisely the FDA’s concern.
An FDA spokeswoman, who did not return our calls at the time of publishing, told the Wall Street Journal that the agency “needs to review genetic tests sold directly to consumers to make sure they are safe and do what they claim to do.” She said the agency issued its warning letter “after the company has repeatedly failed to address specific concerns outlined by the agency since July 2025.”
Dr. Besser explained that this device, or test, isn’t unlike a new drug that enters the market. Drugs are promoted for health and for treatment, which means they need approval and licensure by the FDA. These drugs, take for instance a prescription cholesterol medication, make health claims for the treatment and/or prevention of disease, therefore subjecting them to full FDA oversight, review, validation, and approval. Pregnancy tests or home HIV tests are prime examples – all FDA approved before public sale to consumers because of their diagnostic features. But something like a vitamin, or even diet pills, will not have any FDA approval. They can not be marketed as a product that prevents or treats any health issue; and if they do, that’s where the FDA steps in.
“At this time, we can only provide the following statement from 23andMe,” said a spokesperson for the company when we contacted them this afternoon.
“We have received the warning letter from the Food and Drug Administration. We recognize that we have not met the FDA’s expectations regarding timeline and communication regarding our submission. Our relationship with the FDA is extremely important to us and we are committed to fully engaging with them to address their concerns.”
The FDA’s letter suggested that 23andMe had dismissed previous requests by the agency by assuring them the information they sought was in progress. In a letter dated January of this year, the company said they were “completing the additional analytical and clinical validations for the tests that have been submitted” and “planning extensive labeling studies that will take several months to complete.” When asked about the status of these tests and studies, 23andMe declined any further comment.
Dr. Besser stands strongly by the FDA’s role in keeping consumers safe where their health is concerned. This action by the FDA, he says, “is for our protection.”
Some people are urging friends and family to buy the kits quickly, while they still can. As of this publishing, the kits were available for $99 with a discount for multiple purchases. However, Dr. Besser advised that if the company doesn’t comply with the FDA’s demands by the specified window of time, the letter cites 15 days from November 22 publication date, it could be bad for business. “If they continue to sell, the product may be confiscated.”
Also Read:
The Cotton Ball Diet is Trending Amongst Young Girls
Raw McRib Meat Doesn’t Look Anything Like Ribs or Meat
Dietitians Speak Out in Support of the FDA’s Trans Fat Ban
images via 23andme.com